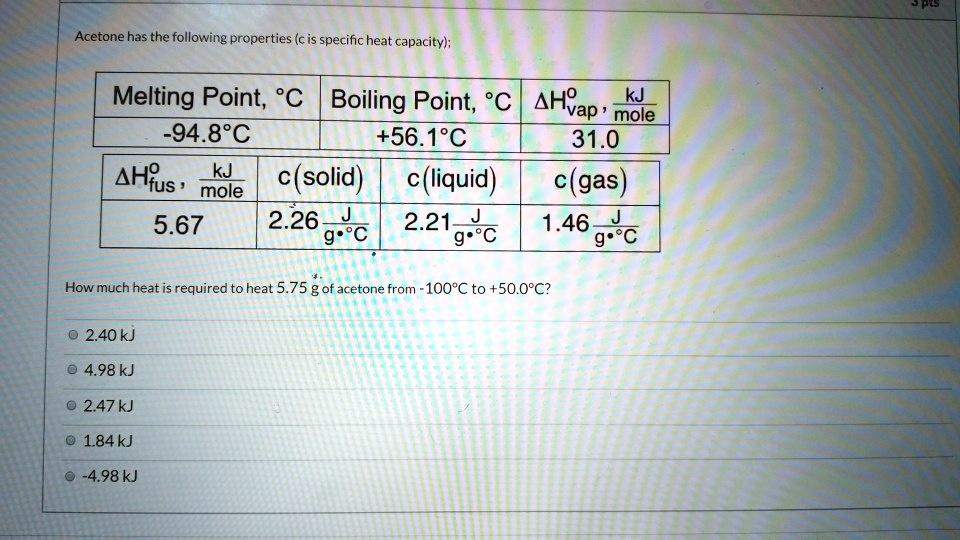
Acetone heat capacities: (c L and c G ) for the molecular liquid and... | Download Scientific Diagram

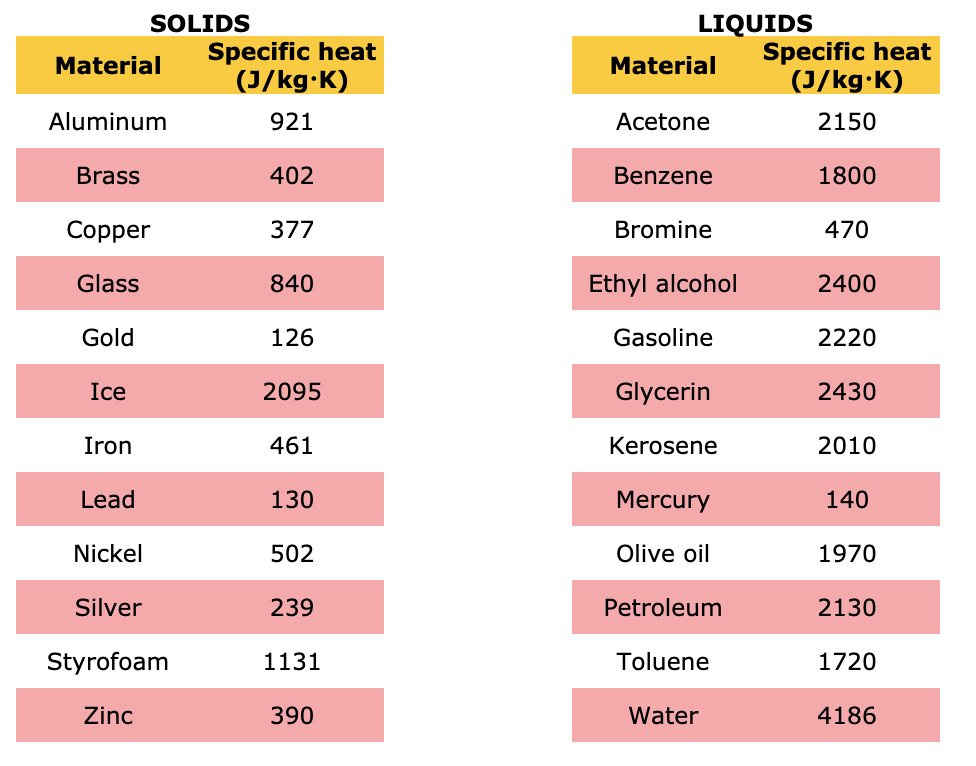
SOLVED: A solid substance has a mass 0f 250.00g It is cooled by 25.00*C and loses 4.937kJ of heat What is the specific heat capacity of the substance in 9.PC [APP 2
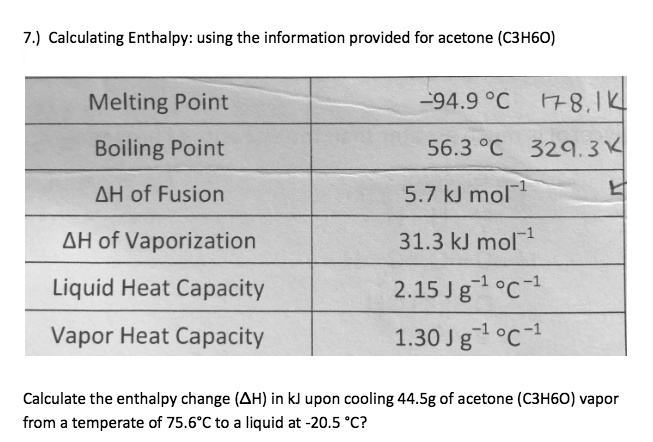
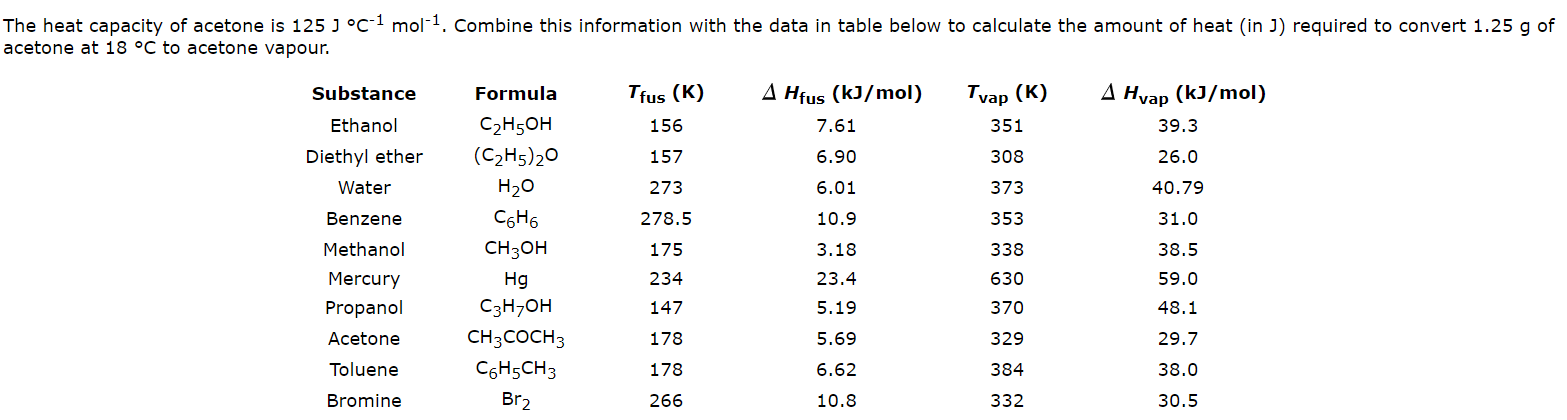
SOLVED: 7.) Calculating Enthalpy: using the information provided for acetone (C3HGO) Melting Point Boiling Point AH of Fusion 94.9 %€ +7-8,1444 56.3 "C 329.34 5.7 kJ mol-1 AH of Vaporization 31.3 kJ
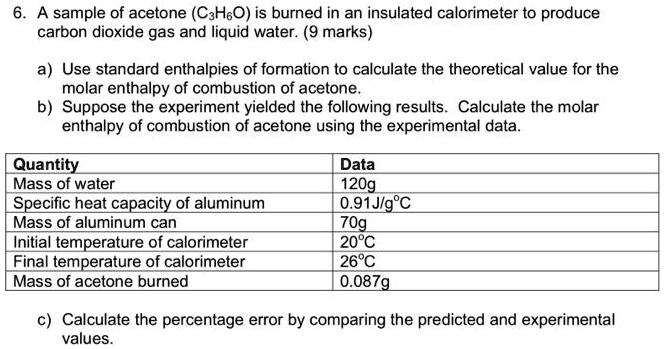
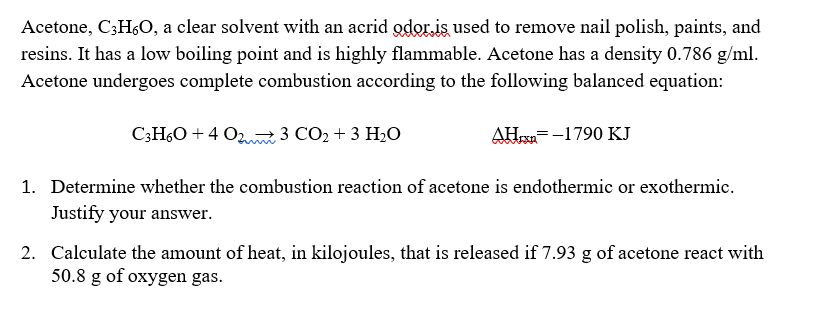
SOLVED: A sample of acetone (CaHsO) is burned in an insulated calorimeter t0 produce carbon dioxide gas and liquid water. (9 marks) Use standard enthalpies of formation to calculate the theoretical value

Heat of vaporization of acetone. Simulation data: • this work, AUA4... | Download Scientific Diagram

Using the given data and calculate the enthalpy of formation of acetone(g). Bond enthalpy of : C - H = 415 ; C - C = 350 ; (C = O) =